Introduction
The prevalence of congenital anomalies of the coronary arteries without clinical manifestations and hemodynamic complications ranges from 0.21% to 5.79% in the general population based on the results of selective coronary angiography (CAG), computed tomography angiography (CTA) and autopsies.1 An anomalous origin of the left circumflex artery (AOLCx) from the right sinus of Valsalva is the most common anomaly with a prevalence of about 0.39%, which in itself often has no clinical significance.2 This anomaly is considered a benign anatomical deviation, but cases of both minor and serious cardiovascular complications have been described: angina pectoris, myocardial infarction, and less often sudden cardiac death.3,4 If in cases when aortic valve replacement (AVR) is needed in a patient with abnormal LCx, the valve prosthesis ring after implantation can cause compression of the artery and therefore may cause myocardial infarction in the early postoperative period.5 This case demonstrates our practice of successful AVR with aortic root enlargement by Nicks in a patient with OALCx from the right coronary artery (RCA).
Case report
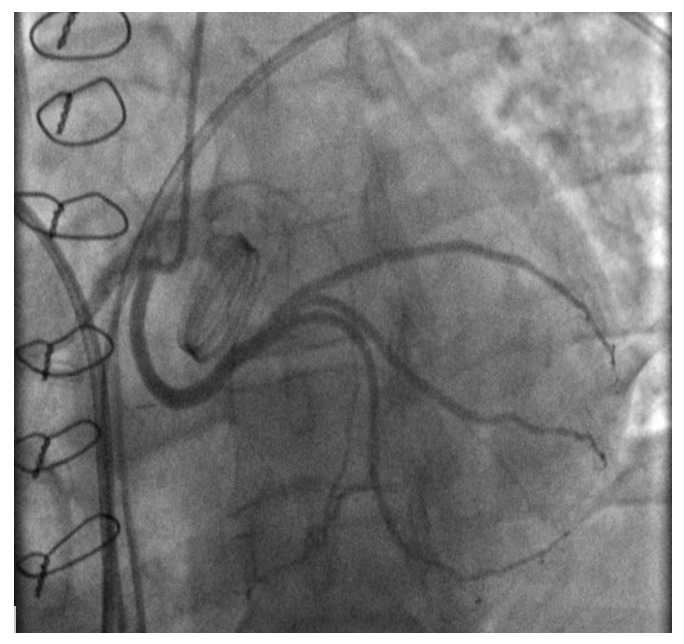
A 45-year-old male (height 168 cm, weight 95 kg, body mass index 33.7, body surface area 2.04 m2) with chest pain was admitted via ambulance to the clinic at his place of residence. During the examination, the patient had a paroxysm of ventricular tachycardia, stopped by medication. According to the ECG data, signs of hypertrophy of the left ventricle (LV) with subepicardial damage to its anterior parts were revealed. After performing thrombolysis (Actilyse (Boehringer ingelheim pharma, GmbH & Co.KG, Germany) 100 mg for 90 minutes), the patient was transferred to our Center for the further treatment. At the time of admission, there was a significant elevation of troponin I levels to 101 ng / ml with progressive decreasing in the follow. Coronary angiography showed no hemodynamically significant stenosis of the coronary arteries, but an anomalous origin of the left circumflex artery from the RCA was noted (Fig.1B). Myocardial injury was regarded as a type 2 myocardial infarction due to ventricular tachycardia paroxysm. Echocardiography revealed severe aortic stenosis with an aortic valve area 0.88 cm², a peak acceleration of blood flow of 4.2 m/s, an average pressure gradient of 42 mmHg, and the diameter of the AV fibrous ring 21 mm. LV hypertrophy was noted: posterior and interventricular septum of 18 and 15, respectively. Also, no regional areas of contractility disorders were noted. Taking into account the acute stage of type 2 myocardial infarction and severe aortic stenosis surgical treatment were postponed for 4-6 weeks. During rehospitalization for the surgical treatment CTA was performed to clarify the anatomical features of the aortic root and coronary arteries (Fig.1A).
After median sternotomy, cardiopulmonary bypass was established via proximal aortic arch and right atrial cannulation, and systemic cooling was initiated targeting a nasopharyngeal temperature of 32 °C. Following aortic cross-clamping and cardiac arrest achieved by retrograde blood cardioplegic perfusion to the coronary sinus. After the mobilization of the aortic root and ascending aorta the anomalous LCx was identified at its origin of the right coronary button coursing down toward the noncoronary sinus of Valsalva (Fig.2A). LCx was mobilized from the side of its origin till the middle third of the left coronary sinus (the zone where LCx is located 15-20 mm below the left coronary artery). After aortotomy, coarse grained calcified unicuspid aortic valve was revealed. Upon direct measurement of the aortic valve annulus with standard calibers, the diameter of the aortic valve fibrous ring was 21 mm. A patient-prothesis mismatch and possible damage to the anomalous LCx were the main reasons to make a decision toward performing AVR with an aortic root enlargement by Nicks (Fig.2B) The aortotomy was extended through the non-coronary sinus and the fibrous ring of the AV to the base of the anterior leaflet of the mitral valve. A wedge-shaped bovine pericardium patch 30x50 mm (KemPeriplas-Neo Xenopericardial, Kemerovo, Russia) used to repair of aortic root and aortic valve fibrous ring. Further, a bicuspid mechanical prosthesis Medtronic Open Pivot AP360 23 mm (Medtronic plc, Minneapolis, Minnesota, USA) was implanted into the aortic valve position with 19 “U”-shaped sutures on Teflon pads (PremiCron 2/0): 9 sutures were located on the aortic side, 6 on the left ventricular side, 4 on the outside of the xenopericardial patch). Postoperative transesophageal echocardiography confirmed excellent prosthesis valve function, and no regional disorders of the LV were revealed.
Postoperative coronary angiography revealed a patent LCx without compression by the prosthetic valve (Fig.3). Control echocardiography showed no significant changes in the size and contractility of the LV; there was a moderate regression of LV hypertrophy compared to preoperative data, and a normally functioning mechanical aortic prosthetic (a peak gradient of 20.1 mmHg, a peak acceleration of blood flow of 2.2 m/s). The postoperative course was uneventful.
Discussion
An anomalous origin of the left circumflex artery from the right sinus of Valsalva or right coronary artery with a retroaortic course is a known coronary anomaly that usually does not cause clinical symptoms.5 In patients with AOLCx the risk of myocardial ischemia due to insufficient myocardial protection and compression by the valve prosthesis ring of anomalous LCx increases during AVR.5 To prevent possible complications due to traumatization of anomalously located LCx when stitching the fibrous ring of the aortic valve. Additional imaging methods should be used for assessing the anatomy of the aortic root and coronary bed. In our case, CTA with three-dimensional reconstructions of aorta and coronary arteries (Fig.1 A.), as a minimally invasive and maximally informative research method, was the most preferable diagnostic tool. In cases where the abnormal LCx originating from the RCA is located retroaortically, it is necessary to take into account its possible ligation during stitching of the fibrous ring of the aortic valve and/or its compression by the ring (sewn cuff) of the prosthesis after implantation of the latter in the AV position.5 Some authors recommend implantation of smaller prosthesis in such cases.6 However, in our case, taking into account the ratio of diameter of the fibrous ring of aortic valve and the surface area of the patient’s body, implantation of a small prosthesis would inevitably entail the development of a patient-prosthetic discrepancy, and therefore it was decided to implant an aortic prosthetic valve with preliminary mobilization of abnormal LCx and performing aortic root enlargement by Nicks. The mobilization of abnormal LCx provided us full control during the aortic root enlargement and stitching of the fibrous ring of the AV, as well as the opportunity to assess the compression of the artery after implantation of the prosthesis. Our case is unique in that it illustrates the possible AVR with a prosthesis of optimal size with preliminary mobilization of the abnormal artery and aortic root enlargement. The successful outcome in this case was the result of preoperative imaging techniques, which made it possible to assess the anatomical features of the abnormally formed coronary bed and the relationship between the coronary arteries and the structures of the aortic root. In cases where the mobilization of abnormal LCx is technically impossible for the prevention of myocardial damage during AVR and/or MVR coronary artery bypass surgery of the abnormal arteries is the method of choice, however, this provision requires additional research.6
Conclusions
This case underscores the critical importance of comprehensive preoperative imaging, specifically CTA with 3D reconstruction, for planning aortic valve surgery in patients with coronary anomalies. The combined strategy of meticulous anomalous LCx mobilization and aortic root enlargement by Nicks technique allowed for the safe implantation of an optimally sized prosthesis, avoiding both coronary compression and patient-prosthesis mismatch. This approach can be considered a viable surgical solution for similar challenging anatomies.
_three-dimensional_reconstruction_of_.png)
_an_anomalous_origin_of_the_left_circumflex_artery_from_the_right_.png)
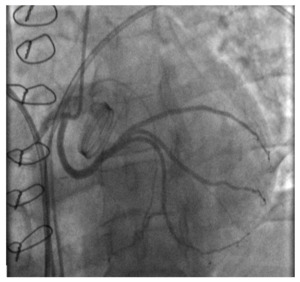
_three-dimensional_reconstruction_of_.png)
_an_anomalous_origin_of_the_left_circumflex_artery_from_the_right_.png)