Introduction
Ventricular septal defects are the most common congenital heart defects. They account for approximately 20% of all forms of congenital heart disease caused by an isolated lesion. When associated with multiple congenital heart defects, the incidence rises to 40%. Perimembranous ventricular septal defects are the most common form (70%), followed by muscular (15%–20%) and sub arterial (5%) ventricular septal defects.1 Depending on the size of the defect, pulmonary hypertension may develop even as early as 18 months to 2 years of age if a large ventricular septal defect is left unrepaired.2
Smaller infants with muscular ventricular septal defects present inherent limitations regarding both surgical and percutaneous device closure, so a hybrid approach, also called “perventricular closure,” has been introduced.3 The initial descriptions included six infants and seven minipigs and showed encouraging results in both cases, without the need to utilize cardiopulmonary bypass.3,4 Muscular ventricular septal defects are a challenging problem in neonates and infants when they present with significant congestive heart failure from interventricular shunting. However, with careful adjustments to the technique, most of the defects can be closed.5
Case Summary
A 4-day old male neonate was transferred to our neonatal unit from the local hospital for continuation of care. He had been diagnosed at the 22+6-week scan with a complete atrioventricular septal defect consisting of a small ventricular septal defect and a large ostium primum septal defect. (Supplementary Material 1) An amniocentesis yielded normal karyotype results. The neonate was born via caesarean section secondary to previous caesarian at 40 weeks of gestation with a birth weight of 4350 g. He was transferred to our hospital due to desaturations and central cyanosis.
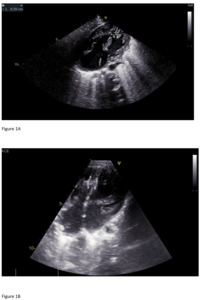
At 20 days of age, he started to have episodes of tachypnea and diaphoresis during feeding. On day 26 of life, he underwent pulmonary artery banding. The postoperative echocardiogram showed common atrioventricular canal type A, loose pulmonary artery banding with a mean pressure gradient (PG) of 17.5 mmHg (Figure 1A), a large mid- muscular ventricular septal defect measuring 5.8–6.7 mm with bilateral flow, a small inlet ventricular septal defects measuring 2 mm, a large ostium primum atrial septal defect, and a large ostium secundum atrial septal defect. There was difficulty weaning him off the ventilator and he required inotropic support. Therefore, on day 38 of life he underwent cardiac catheterization (Supplementary Material 2). The procedure revealed the following:
-
A large muscular ventricular septal defect after pulmonary artery banding, with mean Pulmonary Artery Pressure of 37/10 mmHg (mean 20 mmHg), Qp/Qs of 1.3, and Pulmonary Vascular Resistance of 1.46 Woods Units.
-
A right ventricle with good contractility.
-
A moderate size muscular-type ventricular septal defects with smaller outlets toward the right ventricle.
-
A large atrial septal defect.
-
Moderate reversible pulmonary hypertension.
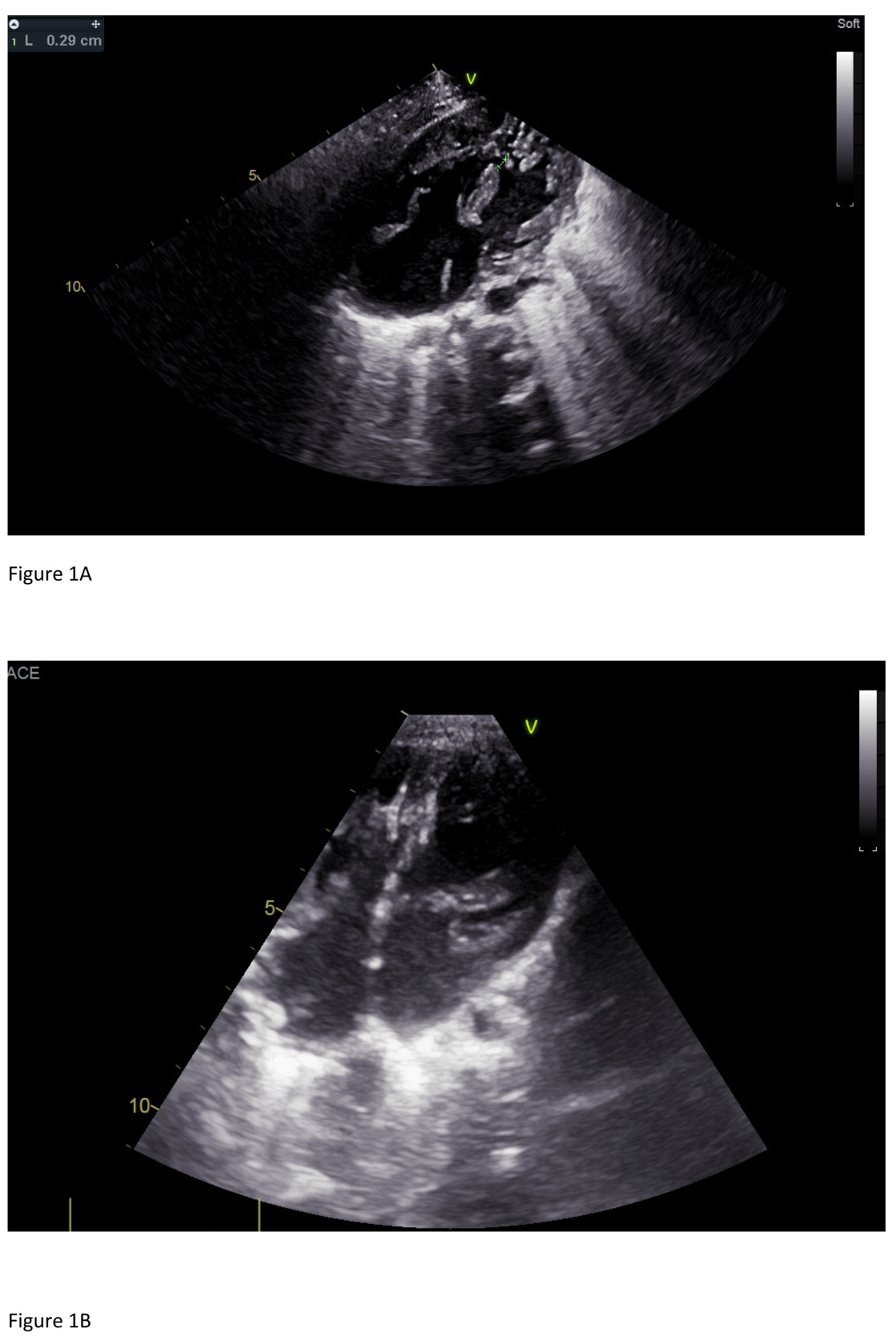
He underwent a new hybrid cardiac operation with patch closure of the atrial septal defect, closure of the VSD with a device, repair of the left and right atrioventricular valve. The device was positioned after right atriotomy and under direct vision, through the tricuspid valve. The device had a diameter of 5mm. An Amplatzer Duct Occluder II (size9-2-6-4) was used with disc diameter of 12mm, a waist diameter of 6 mm, and a waist length of 4 mm (Figure 1B). A device charging system was used but with a short 6 Fr sheath as a release system.
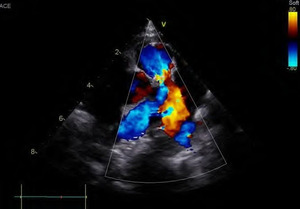
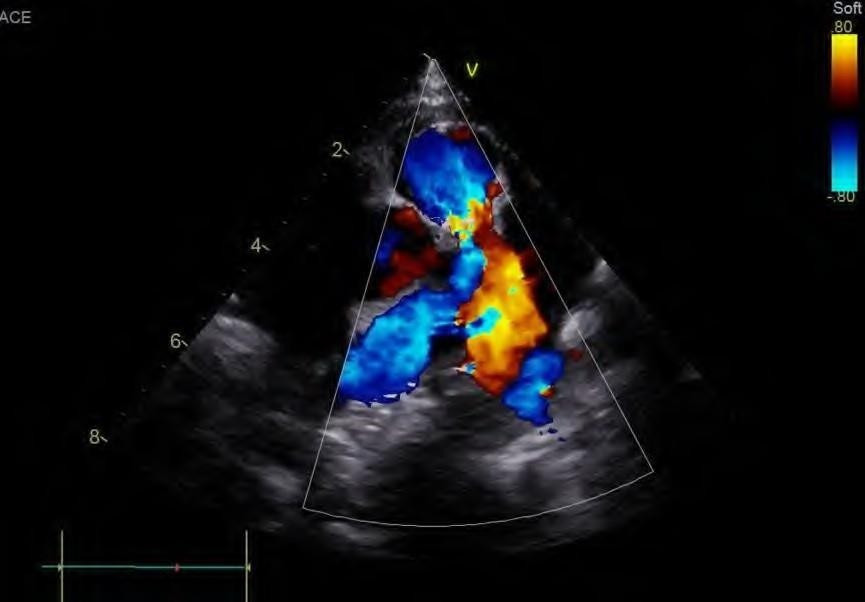
The final echocardiogram showed Pulmonary Artery banding with a max PG of 35 mmHg (mean 17.6 mmHg), small residual flow from the left to right by the ventricular septal defect closure device, and a second apical muscular ventricular septal defect with left-to-right flow with a PG of 24 mmHg, 1+/4+ regurgitation of the left atrioventricular valve with a PG of 75 mmHg, and an intact atrial septum. (Figure 2)
After the intervention, he remained well, and he was able to establish feeding.
Discussion
As early as 1999, perventricular and interventional ventricular septal defects closure had proved effective even in small infants (3.2–8.9 kg body weight).6 In one 8-year study, the perventricular approach for mostly muscular ventricular septal defects was successful in 88.5% of device placements, even with large defects in infants 3.5–14 kg.7 Similarly, in larger cohorts of infants from 25 days to 8.9 years of age weighing 4–12.9 kg, there have been success rates of up to 90%.8–10 Moreover, when a muscular ventricular septal defect is a residual lesion of a more complex congenital heart disease, the perventricular approach has been used with shorter procedural times and good results. Successful device implementation by using the perventricular approach has been reported in isolation and in conjunction with other hybrid procedure. The hybrid procedure has been performed on children with a larger weight range (> 3 kg and has a good safety profile. As in our case, collaboration between interventional and surgical teams is required.4–8 In small children < 15 kg, the hybrid perventricular approach provides an excellent alternative access to the heart, especially in low birth–weight infants, to prevent hemodynamic instability or in small children requiring large delivery sheaths.9,10The hybrid perventricular technique not only reduces the risk of significant complications compared with the conventional surgery, but also produces non-inferior results compared with transcatheter occlusion in selected patients with ventricular septal defect.
Conclusions
The hybrid perventricular approach provides a safe alternative operative choice, even in small patients with complicated congenital heart disease.
Lessons learned
Ventricular septal defects are the most common congenital heart defects. Muscular Ventricular septal defects are a challenging problem in neonates and infants when they present with significant congestive heart failure from interventricular shunting. However, with careful adjustments to the technique, most of these can be closed even in association with more complex congenital heart defects.