Introduction
Pancreatic cancer, most often presenting as pancreatic adenocarcinoma (PDAC), is a highly aggressive malignancy with poor prognosis, reflected by a 5-year survival rate of only 10%.1 It is the seventh leading cause of cancer-related death worldwide,2 and despite advances in treatment, surgical excision remains the only potentially curative option for this type of cancer.3 Genetic factors also play a significant role, with pathogenic variants in BRCA2 being the most common high-risk mutations, found in 1.4% to 7% of unselected pancreatic cancer patients.4 On the other hand, breast cancer is the most prevalent cancer globally and a leading cause of cancer mortality.5 Locally advanced breast cancer, a subset of non-metastatic tumors, presents a significant clinical challenge due to its high relapse rate and poor outcomes.6 While rare, synchronous cancers, such as concurrent pancreatic and breast malignancies, may be discovered during the diagnostic process for a primary cancer,7 complicating the clinical management and prognosis. In this study, we present a rare case of a patient with two primary malignancies as well as the diagnostic and therapeutic challenges regarding the proper management of the patient.
Case Presentation
A 77-year-old Caucasian female was referred by a primary care physician from a health center to the Department of Internal Medicine at our tertiary hospital due to obstructive jaundice and newly diagnosed diabetes mellitus. Regarding her past medical history, she has been suffering from depression and is a current smoker with a 30-pack-year history. The patient complained of anorexia and a weight loss of 15 kg over the last three months. Her family history consists of her father who died from lung cancer.
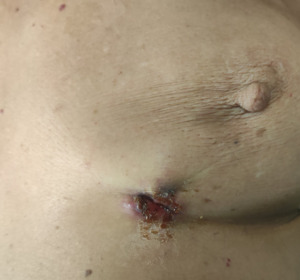
On physical examination, the patient’s skin was yellowish, and an ulcerated mass was present on her left breast (figure 1). Blood tests revealed the following: total bilirubin was 20 mg/dl (normal range: 0.2-1.2 mg/dl), γ-GT 224 U/L (normal range: 12-64 U/L), and ALP 232 U/L (normal range: 40-150 U/L), with a slight elevation in liver enzymes—SGOT was 88 U/L (normal range: 5-34 U/L) and SGPT 112 U/L (normal range: 0-55 U/L). Tumor markers were elevated: CA 19-9 was 2669 U/ml (normal range: 0-37 U/ml), CEA was 66.85 ng/ml (normal range: <10 ng/ml), and CA 15-3 was 42.1 U/ml (normal range: 0-30 U/ml).
During her hospital stay, a CT scan of the brain, thorax, and abdomen was performed, revealing six nodules in the lung parenchyma, the largest of which was 7.4 mm, and a 2.7 cm solitary mass in the left breast. Her abdominal CT scan revealed intrahepatic duct dilatation, a solitary non-enhanced 3.5 cm mass in the pancreas with no signs of portal vein invasion, and a 3.4 cm mass in the left adrenal gland (figure 2). A magnetic resonance imaging, magnetic resonance cholangiopancreatography (MRI/MRCP) performed characterized the lesion in the left adrenal gland as an adenoma. Dilatation of the common bile duct (CBD) (1.7 cm), along with intrahepatic duct dilatation and a pancreatic mass, was also noted.
The patient underwent endoscopic retrograde cholangiopancreatography (ERCP) with placement of a plastic stent, without CBD brushing. Regarding the solitary breast mass, an ultrasound-guided core biopsy was performed. The core biopsy of the breast mass revealed a Grade III invasive ductal carcinoma (non-specific type). Perineural invasion and a mitotic count of >10 mitoses per 40 HPF were present. Immunohistochemical testing showed the following results: ER (+), PR (+), CerbB2 +2, Ki-67 20-35%. The HER2/neu gene was also evaluated using the silver in situ hybridization (SISH) technique, and the result was negative.
Regarding the investigation of the pancreatic mass, the patient underwent endoscopic ultrasound with fine-needle biopsy (FNB). The pancreatic tumor did not invade the portal vein or superior mesenteric artery, and its greatest diameter was found to be 2.8 cm. The biopsy results revealed the presence of a poorly differentiated pancreatic ductal adenocarcinoma. Immunohistochemical testing showed the following results: CK7 (+), CK20 (-), ER (-), PR (-), CerbB2 +2, Mammaglobin (-), and GCDFP-15 (-).
The patient was discharged with a recommendation for new blood tests once the biopsy results became available. Three weeks later, the patient’s laboratory tests showed that her total bilirubin was 0.5 mg/dl, CEA was 14.9 ng/ml, and CA 19-9 was 88.5 U/ml. A new CT scan of the thorax and the abdomen was performed and showed no disease progression. The nodules of the lung remained stable with absence of abnormal axillary lymph nodes.
The case was discussed in a MDT meeting. Given that the patient was diagnosed with a resectable pancreatic cancer and locally advanced breast cancer, the MDT recommended upfront surgery for both cancers: pancreatoduodenectomy (PD) and lumpectomy.
Surgical technique
An upper midline incision was used for better exploration of the abdominal cavity. The peritoneum and liver were assessed for the presence of metastases, and the pancreas was evaluated for tumor resectability. The texture of the pancreas was hard and the pancreatic duct diameter was 4mm.
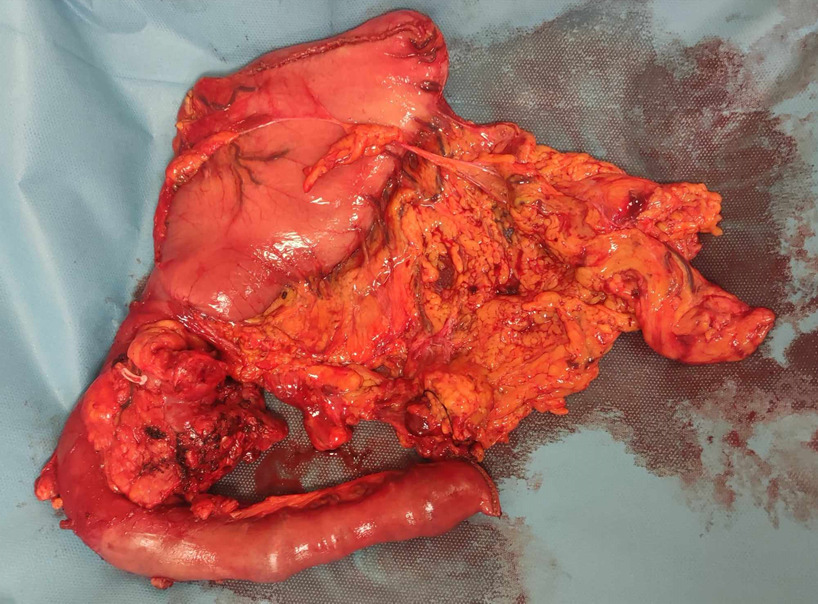
After the excision of the PD specimen, the breast team performed a lumpectomy of the breast mass (figures 3, 4). No sentinel lymph node biopsy was performed. A drain was placed, and the skin was closed with non-absorbable sutures. For reconstructions, we used a pancreaticojejunostomy in the form of an end-to-side anastomosis, specifically using the Blumgart technique. A pancreatic plastic stent was also used. We then performed a hepaticojejunostomy in an end-to-side anastomosis fashion using interrupted absorbable sutures (PDS 4/0) and a gastrojejunostomy with staplers. Two drains were placed. The patient was transferred to the Surgical Assessment Unit for recovery after the operation.
Postoperative course
The patient was transferred to the ward on postoperative day (POD) 3. On POD 5, the nasogastric tube was removed, and oral intake was initiated. The surgical drains were negative for amylase on POD 3, POD 5, and POD 7. On POD 8, the output fluid from the drain was milky-colored, with a triglyceride content of 350 mg/dl, leading to a diagnosis of a chyle leak. As a result, a somatostatin analogue, parenteral nutrition, and dietary restrictions were initiated. The patient was discharged one week later without a surgical drain, and the chyle leak was classified as Grade B.
Pathology report
The histology specimen revealed a 3.5 cm high-grade pancreatic ductal adenocarcinoma (non-specific type) with the presence of squamous metaplasia (25-30%) and invasion of the muscular layer of the duodenum. Tumor emboli, as well as vascular and perineural invasion, were present. Pancreatic intraepithelial neoplasia 3 (PanIN-3) was also noted. Regarding lymph node status, one lymph node out of twenty-six was positive. Immunohistochemical testing showed the following results: ER (-), PR (-), mammaglobin (-), CK 5/6 (+), p63 (+), and p40 (+). The breast lesion was a 2.7 cm Grade III invasive ductal carcinoma (non-specific type) with ulceration of the skin. High-grade comedo ductal carcinoma in situ (DCIS) was also present. The excision of the tumor was complete. Immunohistochemical analysis showed the following: ER (+), PR (+), c-erbB2 (2+), and Ki-67 at 30-35%. Results of the two tumors are summarized on table 1. TNM staging for pancreatic and breast cancer was T2N1 and T4bNx, respectively.
The patient was discussed again in the MDT meeting after the histopathology report was available. Due to the presence of an ER(+), PR(+) breast cancer hormonotherapy was suggested along with whole breast radiation, a boost dose of the tumor bed and radiation of the axilla region. Regarding the pancreatic cancer, chemotherapy consisting of gemcitabine and capecitabine was proposed in an attempt to combine therapeutic target against both types of cancer.
Discussion
We present a case of a patient who was found to have two synchronous malignant neoplasms of the pancreas and breast following an investigation for painless obstructive jaundice. A firm and ulcerated breast mass was discovered on physical examination and was confirmed to be invasive ductal carcinoma after an ultrasound-guided core biopsy. Management of a locally advanced breast cancer as in our case, is rather challenging. Given that our patient’s tumor is clinically T4, as there is skin ulceration, neoadjuvant chemotherapy is indicated as it is mentioned in the NCCN guidelines.8 After proper restaging, the patient could undergo breast conserving surgery (lumpectomy with sentinel lymph node biopsy -SLNB) and based on the results of SLNB, axillary lymph node dissection may be indicated if positive sentinel lymph node was found. Given that the tumor is ER/PR positive, adjuvant endocrine therapy such as an aromatase inhibitor, is indicated.8 Radiotherapy and specifically whole breast radiation therapy is indicated with a boost to the tumor bed as our patient is at higher risk for recurrence. The presence of synchronous pancreatic adenocarcinoma complicated the management of the patient as it is known that pancreatic cancer is an aggressive type of tumor with high rates of recurrence and a few number of patients are candidates for resection at the time of diagnosis.9 The main concern was for potential progression of one or both malignancies during a period of neoadjuvant systemic therapy, that would render one or even both malignancies inoperable. Moreover, pancreatic adenocarcinoma is typically the more aggressive of the two types and the outcome of the patients is mostly determined by the progression of the pancreatic cancer.7The presence of synchronous pancreatic and breast cancer is rare. After reviewing the literature, we found only seven studies discussing synchronous pancreatic and breast cancer, one of which was a literature review.7,10–15 Of these seven papers, only four patients were found to have undergone both pancreatectomy and mastectomy, similar to our case.7,10–12
In three cases, pancreatic resection did not take place due to presence of unresectable pancreatic cancer at the time of diagnosis.13–15 Regarding patients with resection of both cancers, the first patient, reported by Ofri et al,7 was a 61-year-old female with synchronous left breast cancer involving axillary lymph nodes and two pancreatic lesions, one in the body and one in the tail of the pancreas. The patient underwent a total pancreatectomy and mastectomy with axillary lymph node dissection.
The second patient, reported by Nakanishi et al,10 was a 78-year-old female with synchronous left breast cancer involving axillary lymph nodes and pancreatic adenocarcinoma with portal vein invasion. Due to the borderline resectability of the pancreatic cancer, she received neoadjuvant chemotherapy, resulting in a partial response in the pancreatic cancer and stable disease in the breast cancer. Consequently, she underwent pancreatosplenectomy with vascular resections and reconstructions, as well as a mastectomy with axillary lymph node dissection.
The third patient, a 41-year-old female, was reported by Castro et al.11 She had synchronous locally advanced breast cancer and pancreatic adenocarcinoma of the tail. She underwent a modified left mastectomy, and one month later, laparoscopic hand-assisted left pancreatectomy and salpingooophorectomy due to the presence of a BRCA-2 mutation. Radiotherapy to the post-mastectomy region and upper abdomen, as well as endocrine therapy, were also administered.
The fourth patient, reported by Unek et al,12 was a 50-year-old male with synchronous right breast cancer and pancreatic adenocarcinoma of the head. He underwent pancreatoduodenectomy and mastectomy, followed by radiotherapy to the pancreatic region and chest wall, and chemotherapy.
Another case report by Erikoglu et al,16 reported a case of a 55-year-old patient with T2N1 breast cancer who underwent a lumpectomy and axillary lymph node dissection six months after a pancreatoduodenectomy for T3N0 pancreatic adenocarcinoma. The second tumor was detected during follow-up due to the presence of a breast mass. The patient did not receive adjuvant chemotherapy after the pancreatoduodenectomy due to the patient’s refusal. After resection of the breast mass, she was administered adjuvant chemotherapy followed by paclitaxel, anastrozole, and whole-breast radiation therapy.
Conclusion
Synchronous pancreatic and breast cancer is rare, with only a limited number of case reports addressing this issue. The management of such patients remains controversial, as there is no universally accepted therapeutic plan, and chemotherapy regimens are often unsuitable for treating both cancers simultaneously. Surgery is typically recommended when both tumors are resectable, particularly for pancreatic cancer, as this neoplasm is more aggressive, and resection remains the only potentially curative option.
Conflicts of interest
All authors declare that there is not any conflict of interest.
Sources of funding
No funding sources.


.png)


.png)