Case Report
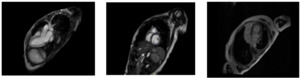
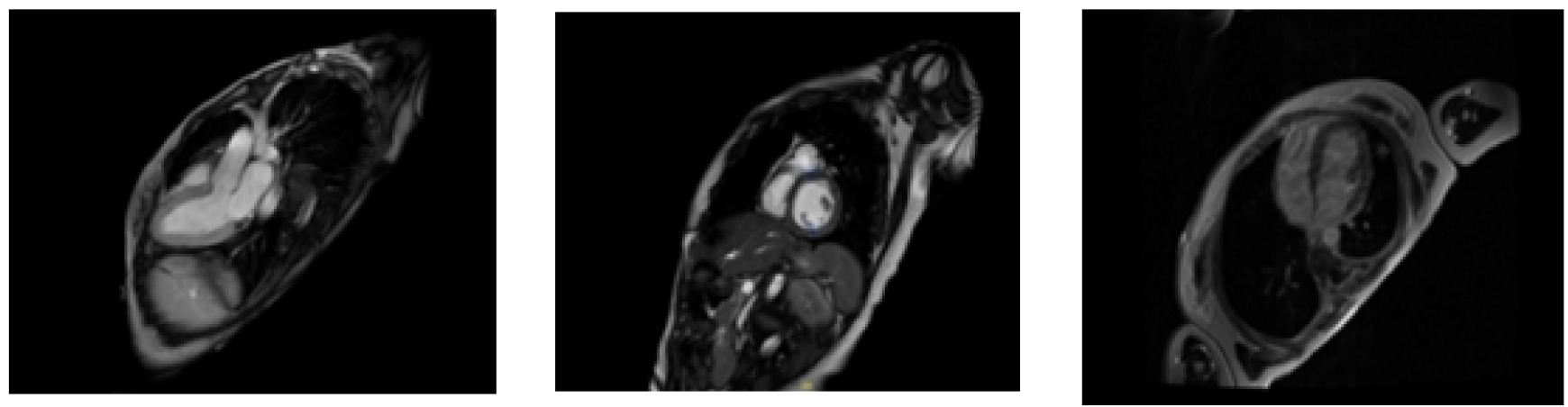
40-year-old female patient with past medical history of anxiety, GERD, heart failure with improved ejection fraction from 25 to 65% after parvovirus infection who initially presented to the ED with chest pain, fever and shortness of breath. Patient was tested positive for COVID 19 infection few days before the ED presentation. In the ED, her D-dimer was normal, troponin 34> 48>75 and then 132>135>198 and delta of 66. Lactic acid 1.5, CRP 4.2, BNP 122 and EKG showed normal sinus rhythm. Patient was admitted to the hospital for observation and was discharged in the next day due to improvement of her symptoms. 2 days after discharge, patient presented again with symptoms and signs of cardiogenic shock as on arrival to the ED, she was hypotensive, hypothermic, tachypneic and hypoxic. She also noted to be cold and clammy. Labs showed lactic acid of 6.6, troponin 17803 which peaked at 18796 and delta of 993. EKG suggestive of PR depression with diffuse ST segment elevation in lead I, 2, aVF, V3, V4, V5. BNP was 3547 and creatinine was normal. Patient also had elevated ferritin, CRP, procalcitonin, markedly reduced platelet count and normal interleukin 6. TTE showed left ventricle ejection fraction of 15% with global hypokinesis, mild drop in right ventricle ejection fraction and moderate pericardial effusion with RA collapse. Patient was admitted to the ICU, intubated, and was started on norepinephrine and milrinone. Impella was placed to provide support however patient continues to deteriorate. Patient became anuric and had hematuria secondary to high speed on Impella support. Her LFTs were trending up and demonstrated signs of hypoperfusion. Patient was evaluated for VA ECMO and ECMO was placed on the next day of Impella placement. During the ICU stay patient developed severe progressive encephalopathy. After 11 days of ICU stay patient was weaned off pressors, extubated and Impella was removed. Patient encephalopathy started to improve however she was found to have bilateral lower extremity profound weakness unclear exact onset which progressed to inability to move her lower extremities. Patient had MRI of thoracic showed extensive enhancement of the proximal cauda equina nerve roots surrounding the conus and extending inferiorly into the lumbar spine. MRI lumbar spine showed there is thickening and rather extensive enhancement of the cauda equina nerve roots beginning in the lower thoracic spine and extending throughout the cauda equina into the sacrum. lumbar puncture showed increased CSF protein with normal WBC’s, glucose, and RBCs which is suggestive of Guillain-Barré syndrome. Patient was treated by IVIG however her lower extremity weakness did not show any quick improvement. Repeat echo 14 days from the onset of cardiogenic shock and presentation showed left ventricular ejection fraction 55% with normal systolic function. Cardiac MRI was done 23 days after presentation which showed EF of 51%, normal left ventricular size, normal right ventricular size and function and no evidence of myocardial inflammation, infiltrative disease, or scarring.
MRI images showed no signs of inflammation and complete recovery 23 days after presentation
Discussion
Multisystem inflammatory syndrome in adults (MIS-A) is an uncommon but severe and still understudied post-infectious complication of COVID-19. Clinically, the disease manifests itself most often 2–6 weeks after overcoming the infection. Young and middle-aged patients are especially affected.
Criteria for Diagnosing MIS-A
A patient aged ≥21 years hospitalized for ≥24 hours, or with an illness resulting in death, who meets the following clinical and laboratory criteria.
Clinical Criteria
Fever (≥38.0 C) for ≥24 hours prior to hospitalization or within the first THREE days of hospitalization and at least THREE of the following clinical criteria. At least ONE must be a primary clinical criterion.
Primary clinical criteria
-
Severe cardiac illness Includes myocarditis, pericarditis, coronary artery dilatation/aneurysm, or new-onset right or left ventricular dysfunction (LVEF<50%), 2nd/3rd degree A-V block, or ventricular tachycardia.
-
Rash AND non-purulent conjunctivitis
Secondary clinical criteria
-
encephalopathy in a patient without prior cognitive impairment, seizures, meningeal signs, or peripheral neuropathy (including Guillain-Barré syndrome)
-
Shock or hypotension
-
Abdominal pain, vomiting, or diarrhea
-
platelet count <150,000/ microliter
Laboratory evidence
-
Elevated levels of at least TWO of the following: C-reactive protein, ferritin, IL-6, erythrocyte sedimentation rate, procalcitonin
-
A positive SARS-CoV-2 test for current or recent infection by RT-PCR, serology, or antigen detection
Conclusion
Myocarditis following MIS-A showed rapid recovery compared to classic myocarditis following viral infection. However, our case showed rapid recovery of cardiac disease, it is essential to emphasize that unrecognized MIS-A has a high mortality rate, so it is necessary to start treatment early as soon as the disease is clinically suspected. The diagnosis of the disease should be based on high clinical suspicion. Treatment should not be delayed waiting for confirmatory lab and imaging results. Treatment options include corticosteroid and IVIG besides supportive treatment specific for the organ involved like cardiopulmonary support in patient with cardiogenic shock.